how many electrons in f orbital|Electronic Orbitals : Bacolod Magnetic Spin, Magnetism, and Magnetic Field Lines. An atom with unpaired . R is a free software project that provides a wide range of statistical and graphical techniques, similar to the S language and environment. R is highly extensible, user .
PH0 · s,p,d,f Orbitals
PH1 · The periodic table, electron shells, and orbitals
PH2 · How many electrons can an orbital of type f hold?
PH3 · How many electrons can an f orbital have?
PH4 · For s, p, d, and f orbitals, how many electrons can each hold?
PH5 · Electronic Orbitals
PH6 · Electron Configuration for Fluorine (F, and F– ion)
PH7 · Electron Configuration for Fluorine (F, and F– ion)
PH8 · Atomic orbital
PH9 · 8.3: Electron Configurations
PH10 · 2.4 Electron Configurations
http://www.indiarace.comAll Videos Courtesy under the respective Race clubs below.Royal Western India Turf Club, Ltd. (rwitc.com)Royal Calcutta Turf Club (r.
how many electrons in f orbital*******This means that the s orbital can contain up to two electrons, the p orbital can contain up to six electrons, the d orbital can contain up to 10 electrons, and the f orbital can contain up to 14 electrons.Magnetic Spin, Magnetism, and Magnetic Field Lines. An atom with unpaired .how many electrons in f orbitalAs shown by the graphs, electrons of the s orbital are found closer to the nucleus .The four different types of orbitals (s,p,d, and f) have different shapes, and one .Learn about the shapes, sizes and capacities of s,p,d,f orbitals, the regions of space where electrons are most likely to be found. Find out how many electrons can s,p,d,f orbitals hold and see examples of electron .The web page is a question and answer forum about the maximum number of electrons that can occupy an f-type orbital. The correct answer is 2, according to the Pauli . For two series, lanthanum (La) through lutetium (Lu) and actinium (Ac) through lawrencium (Lr), 14 f electrons (l = 3, 2l + 1 = 7 m l values; thus, seven orbitals with a combined capacity of 14 electrons) .
Answer link. The f orbital has 7 sub levels with the possibility of two electrons in each suborbital. Therefore, the f orbital can hold 14 electrons.The simple names s orbital, p orbital, d orbital, and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2, and 3 respectively. These names, together with the value of n, are used to .
how many electrons in f orbital Electronic Orbitals The total number of electrons in fluorine is nine. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons in fluorine in specific .
Electronic Orbitals Every subshell has a # of orbits s/p/d/f that can each hold 2 electrons each (one has the opposite spin of the other). The first shell (of all atoms) has 1 subshell of s-orbitals containing 1 s orbital. This means that the first shell can hold 2 electrons. The second shell has 2 subshells: 1 s-orbital and 3 p-orbitals.
Quantum numbers for the first four shells. Calculates number of orbitals and number of electrons in different kinds of orbitals for n = 1 to 4. Explains that only two electrons are .An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball made of rather fluffy material with the nucleus at its centre. As the energy levels increase, the electrons are located further from the . s-orbitals can hold 2 electrons, the p-orbitals can hold 6 electrons. Thus, the second shell can have 8 electrons. The n=3 (third) shell has: The 3s orbital; The 3p orbitals; The 3d orbitals; s-orbitals can hold 2 electrons, p-orbitals can hold 6, and d-orbitals can hold 10, for a total of 18 electrons. Therefore, the formula $2n^2$ holds!
1 Answer. The f sublevel as a whole can hold up to 14 electrons due to the fact that it consists of 7 orbitals, but each one can only hold up to 2 electrons. 2 electrons ---> see below: The f sublevel as a whole can hold up to 14 electrons due to the fact that it consists of 7 orbitals, but each one can only hold up to 2 electrons.
Fourteen would be the maximum number of electrons across an entire f-type sub-shell, but the question only asks about one orbital. answered. 11.9k. The question specifically ask that no.of electron an orbital of f subshell can hold.. As we know that f subshell contain 7 orbital and each orbital can hold maximum 2 electons so correct answer .
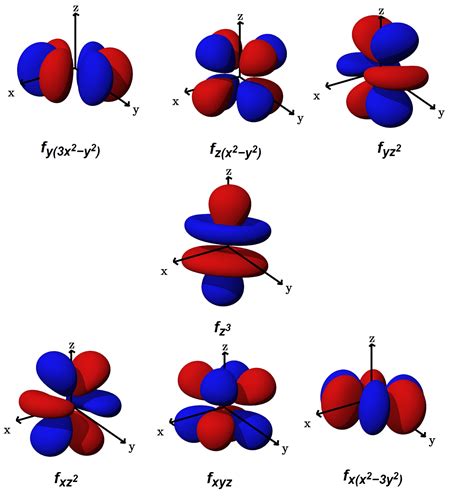
The energy levels are labeled with an n value, where n = 1, 2, 3, .. Generally speaking, the energy of an electron in an atom is greater for greater values of n. This number, n, is referred to as the principal quantum number. Figure 5.7.1 5.7. 1: Different energy levels are numbered by principal quantum numbers n.7f atomic orbitals. For any atom, there are seven 7f orbitals. The f-orbitals are unusual in that there are two sets of orbitals in common use.The first set is known as the general set, this page.The second set is the cubic set, this page and these might be appropriate to use if the atom is in a cubic environment, for instance. Three of the orbitals are common to . The Azimuthal Quantum Number. The second quantum number is often called the azimuthal quantum number (l).The value of l describes the shape of the region of space occupied by the electron. The allowed values of l depend on the value of n and can range from 0 to n − 1: \[l = 0, 1, 2,., n − 1 \label{6.5.2}\]
How many electrons are present in the f block? Flexi Says: There are 7 f orbitals, and each orbital can hold a maximum of 2 electrons, the f block can accommodate a total of 14 electrons. Therefore, there are 14 electrons present in the f block for each element's respective electron configuration. Discuss further with Flexi.Each orbital can hold two electrons. They are also known as atomic orbitals. Atomic orbitals come in different shapes, depending on how much energy and angular momentum is associated with that orbital. .F orbitals are the orbitals that, in total, have the affinity to accommodate 14 electrons in them. The shape of the f orbital is tetrahedral. Though the shape of the f orbital is more complex than the other orbitals, the rule of filling the orbital remains the same as that of p and the d orbitals. The alignment of the electrons is also found to .If an electron has an orbital angular momentum of 7.892 x 10-34 Js, what is the orbital quantum number for the state of the electron? How many valence electrons does promethium have? How many orbitals are contained in the third principal level (n=3) of .
Here, the energy of 4s orbital is less than that of 3d. So, the electron will enter the 4s orbital first and enter the 3d orbital when the 4s orbital is full. The method of entering electrons into orbitals through the Aufbau principle is 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d. The first two electrons of fluorine enter the 1s orbital.

Solution. The subshells s, p, d, and f contain the following number of orbitals respectively, where every orbital can hold up to two electrons maximum: s: 1 orbital, 2 electrons. p: 3 orbitals, 6 electrons. d: 5 orbitals, 10 electrons. f: 7 orbitals, 14 electrons. Was this answer helpful?
10 d orbital electrons: 14 f orbital electrons: Visualizing Electron Orbitals. As discussed in the previous section, the magnetic quantum number (m l) can range from –l to +l. The number of possible values is the number of lobes (orbitals) there are in the s, p, d, and f subshells. As shown in Table 1, the s subshell has one lobe, the p . These are arbitrarily given the symbols px, py and pz. This is simply for convenience; the x, y, and z directions change constantly as the atom tumbles in space. Figure 3: Hydrogen's electron - the 2p orbitals. The p orbitals at the second energy level are called 2p x, 2p y and 2p z. There are similar orbitals at subsequent levels: 3p x, 3p y . The four chemically important types of atomic orbital correspond to values of ℓ = 0, 1, 2, and 3. Orbitals with ℓ = 0 are s orbitals and are spherically symmetrical, with the greatest probability of finding the electron occurring at the nucleus. All orbitals with values of n > 1 and ell = 0 contain one or more nodes.
I just had a pristine Rolex GMT Master II delivered to me from Trusty-time. A true 1;1 replica. I will have to tweek the GMT hand. But please! It is a replica! Andrew was a breeze to work with. Time, about 35 days. But given the SNAFU with shipping everywhere. I ordered a watch from Ukraine in December and it didn't get here until 1/2 way .
how many electrons in f orbital|Electronic Orbitals